Why should you even bother learning about pH when you are doing great despite having snoozed through that dull chemistry class about pH? Healthy skin and pH are closely related, so much so that you really need to pay attention. The health of your skin is decided by the correct balance on the pH scale.
This revolutionary discovery happened in the 1920s when A. Marchionini and his teacher H. Schade identified the acidic nature of the skin. This showed the importance of understanding pH balance and its link with a beautiful skin.
Marchionini and Schade found that the acidic environment on the skin surface prevents the growth of harmful bacteria and fungi. Other scientists later found how chronic alkalization can disrupt the functioning of healthy skin. This article will take you through everything you need to know about skin pH, and we promise it won’t be dull like that chemistry class was!
What is pH anyway?
pH stands for “Potential Hydrogen” or “Power of Hydrogen.” It is your scale of choice if you want to measure the acidity or alkalinity of anything in comparison to distilled water, which is neutral.
The pH scale ranges from 0 to 14. A pH of 7 shows neutrality. It is neither acidic nor alkaline. The further a substance is from neutral on the pH scale, the more alkaline it is. In other words, the higher the number, the more alkaline. Similarly, as we go down from pH 7, the acidity increases. In other words, the lower the number, the more strongly acidic the substance is. Something with a pH of 1 is extremely acidic. We hope this refreshes your chemistry class concepts!
Fun fact 1:
pH doesn’t work like a numerical scale. A jump from pH 7 to 6 seems like a small difference of 1, but it’s actually a 10-fold change in hydrogen concentration. Therefore, a slight change in pH is a significant change.
The pH of some common substances
- Stomach acid – pH 1.5-3.5
- Lemon Juice – pH 2.0
- Tea / Coffee – pH 5.0
- Saliva pH – 5.3-7.8
- Milk pH – 6.8
- Human blood – pH 7.4
- Baking soda – pH 9.0
- Soaps & detergents – pH 9.0-10.0
Important factors influencing the skin pH
Many factors – both internal and external – affect the pH of your skin.
Here’s an official classification of these factors
Endogenous factors
- Age
- Anatomical site
- Genetic predisposition
- Ethnic differences
- Sebum
- Skin moisture
- Sweat
Exogenous factors
- Detergents
- Cosmetics
- Soaps
- Occlusive dressings
- Skin irritants
- Topical use of anti-bacterial products
Fun fact 2:
Ever questioned why your armpits produce odour while your face doesn’t smell of sweat? It is because the pH differs in different anatomical sites. The high pH in the axilla region results in the growth of odour-producing bacteria, hence the armpit odour. Of course, there are some other reasons too.
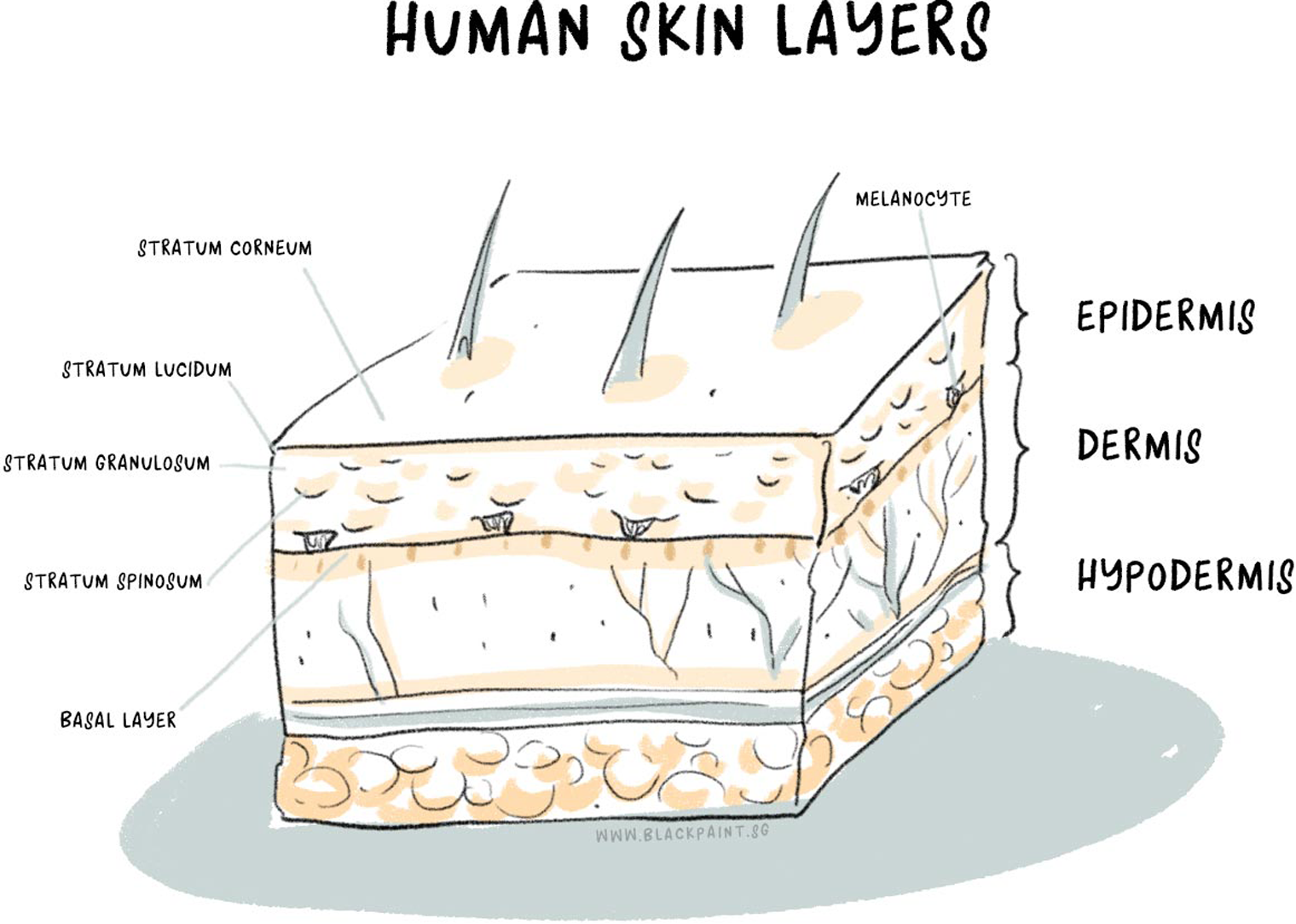
Details of the skin layers
We are all aware of the main organs in the body – the heart, lungs, stomach, kidneys, and others. However, most of us do not think of skin as an organ.
Fun fact 3:
Many professors like to ask this question in a biology or physiology class: What’s the largest organ of the body? Many students get it wrong. It’s not the liver. The biggest organ of the body is the skin.
Human skin regulates the body temperature, permits sensations, and protects us from microbes and toxic elements. It is composed of three layers:
- Epidermis – the top layer
- Dermis – the middle layer
- Subcutaneous fat – the bottom layer
1. Epidermis
This is the topmost layer of the skin and is further composed of four to five layers, namely, (from deep to superficial) stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. Remember stratum corneum; it’ll come up in the later sections of this article with reference to skin pH.
The epidermis performs several important functions, such as generating new cells, protecting the body by contributing to immunity and giving colour to the skin. You read it right- the epidermis determines the skin colour. It has melanocytes which give your skin a dark or a light colour based on the concentration of melanin.
2. Dermis
This layer under the epidermis has its own blood supply to nourish the skin. The dermis has multiple important roles. It contains sweat glands and hair roots. The sweat glands produce sweat to help maintain your body temperature and to excrete electrolytes and toxins.
The dermis also contains sebaceous glands that produce oil. Sometimes the glands produce excess oil, which may lead to acne. Any oily-skin-related issues you may have stemmed from this layer of the skin.
The nerve endings that help you feel hot, cold, rough, smooth, itchy, etc., are also found in the dermis.
3. Subcutaneous fat
The bottom layer of the skin, the subcutaneous fat, attaches the dermis to the underlying muscles and bones. The fat layer provides insulation- it prevents the body from getting too cold or too warm. Another important aspect is its role in protecting our bones and muscles from the impact of bumps and falls.
Normal skin pH
Human skin pH usually is in the acidic range of 4-6. This range is, however, in contrast to about 50% of the literature which shows that skin pH is on average below 5. Studies have also confirmed that individuals with a skin pH below 5 have a better skin condition compared to those who have their pH within the normal range but above 5.
Effect of normal pH on the skin
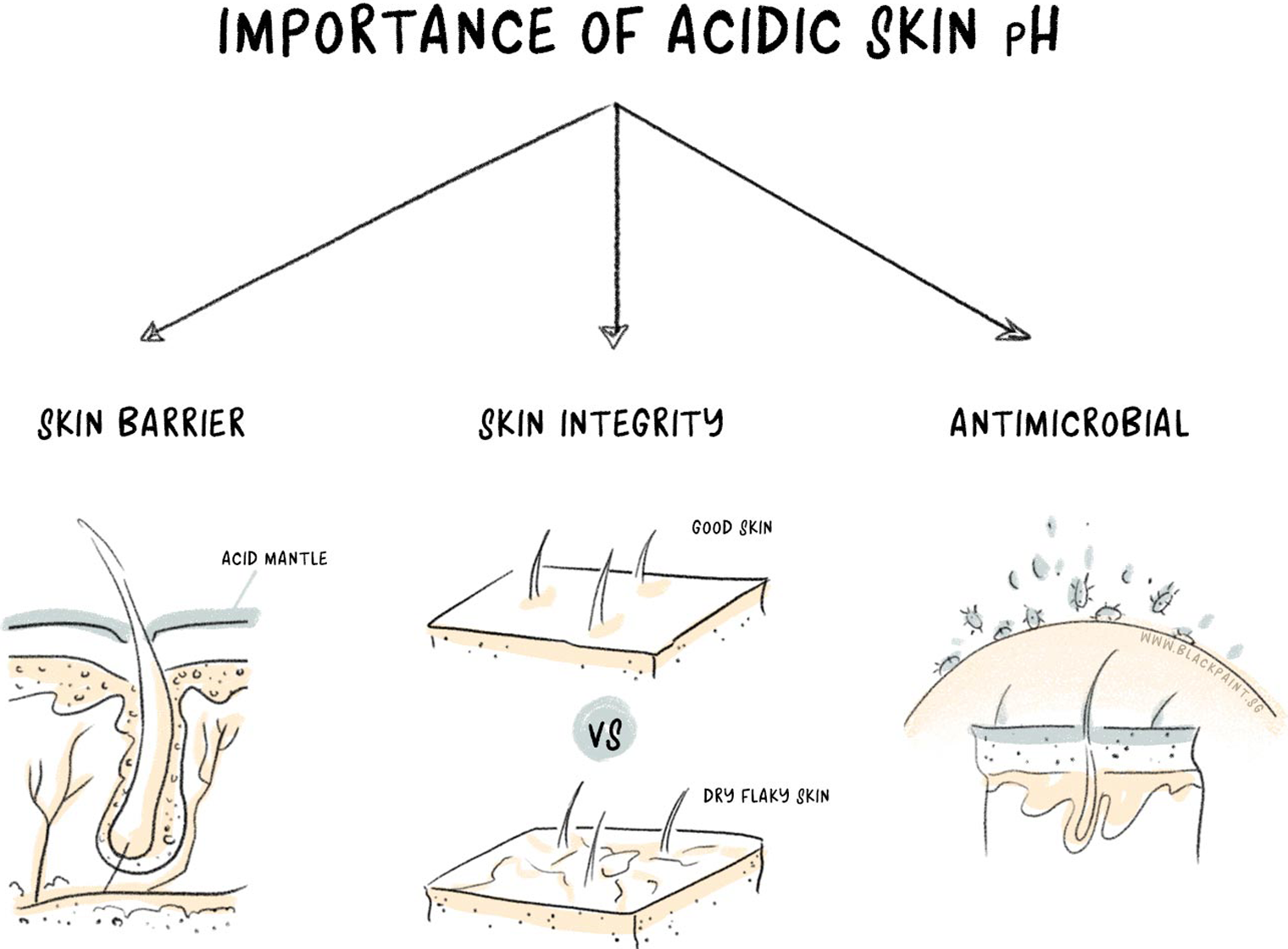
The normal acidic pH of the skin has several important functions:
1. Barrier function
The primary role of our skin is to act as a physical barrier to harmful organisms and toxic substances. The hydrophobic stratum corneum (SC) of the epidermis provides this barrier function. Here’s the twist directing towards pH: Important enzyme activity in the course of SC formation requires an acidic pH.
No acidic pH = improper SC formation
We get this much-needed acidic environment on the skin from sebum and sweat. Sebum released by the sebaceous glands of the skin mixes with sweat to produce the acid mantle – an acidic film on the surface of stratum corneum. The acid mantle creates a fantastic host defence against microorganisms and is important for both barrier functions and microbial defences. This film requires a pH balance of 5.5 to work at its best.
There’s quite a big difference between our internal and external body pH. Our internal pH ranges from 7-9 (alkaline). Pathogenic bacteria that have adapted to the acidic environment of the skin would fail to thrive in the alkaline environment of internal tissues even if they get to break the skin barrier and gain entry to the underlying tissues. See, it’s a protective mechanism 🙂
Fun fact 4:
Our skin is host to more than 500 species of bacteria. Scary or amusing?
2. Skin integrity
As mentioned earlier, an acidic pH is essential for SC formation. This pH level is also responsible for keeping the integrity and cohesion of the stratum corneum. Some science is involved in this skin integrity talk. We’ll try to keep it as simple as possible.
An increase in the skin’s pH leads to an increase in the activity of serine protease enzymes that eventually result in abnormal skin integrity. Additionally, a high pH causes low lipid processing. (Remember the stratum corneum had a hydrophilic nature? That was due to its high lipid profile) and abnormal barrier permeability. Whereas, an acidic pH activates enzymes that are protective of the SC structure and function. Therefore, an acidic pH is necessary for skin integrity.
3. Antimicrobial properties
Your skin has a combination of permanent, temporary and transient bacterial residents. Of these three classes, all bacterial species constituting the normal flora of the skin work best in an acidic environment, whereas pathogenic bacteria thrive at high pH levels. For example, Staphylococcus aureus– the culprit behind eczema, acne, and rosacea – thrives at a neutral pH.
Dermicidin, an antibacterial peptide secreted by sweat glands, requires an acidic pH to induce a bactericidal effect on harmful bacteria. Similarly, there are other substances in the acid mantle, such as nitrates and certain basic proteins, which have antibacterial properties and require an acidic pH to induce that effect.
Fun fact 5:
Microbial organisms contribute up to 3% of our body weight.
The pH levels of skincare products and their effects on your skin
Many external factors like detergents, cleansers, cosmetics, topical antibiotics and occlusive dressings influence the pH of the skin. The negative influence of such products has been reported to play an important role in dermal conditions like acne and contact dermatitis.
What levels of pH are harmful to the skin?
Unbalanced pH levels – whether highly acidic or highly alkaline – are detrimental to the skin. Maintaining the balance is essential. Look for pH-balanced skincare products.
Highly alkaline products, such as soaps with a pH range of 9-11, can deprive your skin of natural fats, demolish hydration, and disrupt the acid mantle. This is one of the reasons why baking soda, which is highly alkaline, is not a good home remedy for your skin. A dry, tight, and/or dull skin is an indication of high alkalinity.
Having highly acidic skin can cause erythema and pimple breakouts. Although it is comparatively rare to have over-acidic skin, acne prone individuals and consumers of lemon cleansers can have such issues. The use of products with a pH lower than 4 can lead to red and irritated skin. If you have acne, the use of skin cleansing products with pH 5.5 may be a suitable choice in preventing and treating pimples.
Warning: Do not buy highly acidic products for a chemical peel effect. Always consult your dermatologist first.
Is 5.5 really an optimum pH for skincare products?
Many cosmetic companies claim that their products are pH-balanced at a value of 5.5 and are, therefore, more suitable for the skin. There has been no research to back up this statement. However, scientific studies do show that on average, individuals with skin pH below 5 have a better skin condition as compared to others. In contrast, many other studies have confirmed that normal skin pH can be anywhere between 4 and 6.
A pH-balanced product is important for the health of your skin, but we cannot say for sure if the 5.5 pH value is optimal – at least not until research proves it.
The case of lemon in the court of dermatologists
Lemons are beneficial to eat; they are a rich source of vitamin C, fibre, and antioxidants. The problem, however, is that most often digital content presents lemon as a cure for every other skin problem.
Before you judge, let us explain.
We love lemons; they are just not the best thing for the skin.
Each lemon remedy looks promising, but most people fail to understand that lemons are highly acidic – far more acidic than our skin pH. Applying lemon juice to the skin can lead to irritation, redness, sensitivity, and even hyperpigmentation. Ironically, some home remedies suggest lemon juice for treating blemishes.
Simple fact: Applying acidic stuff that is out of the normal skin pH range can leave your skin dry, itchy, irritated, and prone to infection.
While not all dermatologists are against applying diluted lemon juice to the skin, some are. Those who vote in favour believe that the highly acidic nature of lemon juice can be changed by mixing it with appropriate products such as egg white. Others simply refuse to take the risk of trying a home remedy that might bring harm.
If you are using a homemade lemon product, make sure it is diluted and that you only leave it on for a little while.
Dermal problems & skin pH
The barrier function of skin imparts resistance against external aggressions. When the pH of the skin is disturbed, the acid mantle is disrupted, giving deep access to harmful bacteria and providing an alkaline environment that lets pathogenic bacteria grow. The result is various skin conditions, such as atopic dermatitis, irritant contact dermatitis, and acne vulgaris.
1. Atopic dermatitis (Eczema)
In a research study analyzing the skin pH of 100 children with atopic dermatitis (AD), pH levels were found to be significantly higher in the eczema skin compared to the healthy skin of the same individuals. Involved areas of skin with intense itching and dryness had an even higher pH.
Disturbed synthesis and maturation of the stratum corneum partly explain the impaired barrier function of skin in AD. The itch induced by AD further damages the surface. Additionally, Staphylococcus aureus colonization is an important pathologic factor. The adhesion of S. aureus with the human skin increases with increasing pH.
2. Irritant contact dermatitis
Individuals prone to irritant contact dermatitis have been shown to have higher pH values when compared with the healthy participants of the research studies. A pH-induced decline in the integrity and barrier function of the stratum corneum further makes the skin prone to injury from solvents, detergents, and mechanical forces.
3. Acne
Skin pH and acne are highly related. Studies have shown that acne breakouts significantly decline at low pH values, whereas severe cases of acne have high skin pH.
A study conducted on acne-prone patients compared the number of pimple breakouts in participants using an acidic syndet bar versus those using conventional alkaline soap. The acidic syndet group had a significant decline in pimple breakouts by the fourth week of application. That is why most acne care products contain salicylic acid and benzoyl peroxide; both play an important role in bringing down the pH of your skin.
Acidic pH and wound healing
Many forms of dermal conditions result in wounds or breaks in the skin. Science has discovered the beneficial role of an acidic environment in wound healing. An acidic environment controls infection, exerts antimicrobial activity, reduces the toxicity of bacterial secretions, and speeds up the epithelialization process. Most pathogens (disease-causing organisms) grow at a pH value of greater than 6 and have their growth inhibited at a low pH.
Does diet impact skin pH?
When it comes to the internal environment of the human body, an alkaline diet is favoured over acid-forming foods. Alkaline foods are naturally high in minerals and vitamins that are needed for beautiful skin. A diet rich in beta-carotene, vitamin A, C, and E, zinc and omega-3 fatty acids is bound to have positive effects on your skin.
Consuming green veggies, low sugar fruits, good fats, nuts, seeds, herbal teas, and lots of water while limiting acid-forming foods like sugar, processed grains, and alcohol can help keep the pH well balanced.
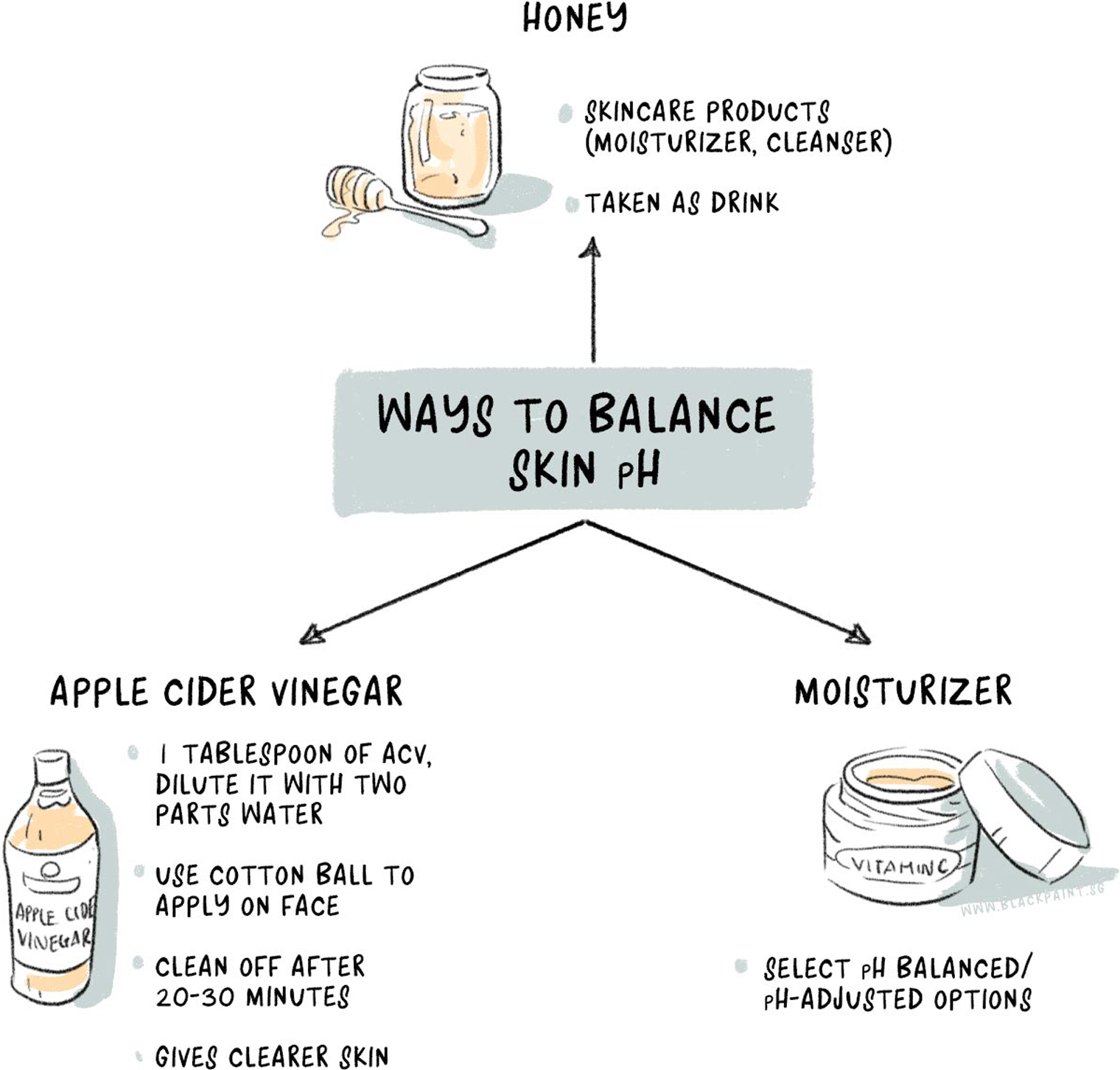
3 ways to balance skin pH levels
A balanced skin pH is important for beautiful skin; no doubt about that. Factors like over-washing, use of soaps, extended sun exposure, use of tap water that has high hardness, and an acidic diet can cause an imbalance in the skin’s pH. Use these three products to restore the pH of your skin.
Honey
Honey makes an excellent choice for a cleanser and has been known for its therapeutic benefits for a long time. Instead of using products with expensive price tags and a bad pH, you can use honey in its raw form as a moisturizer, cleanser, and even an antibacterial agent. Honey is a humectant, which means it keeps water locked into the skin and prevents dryness.
Apple cider vinegar
Apple cider vinegar is considered to be a magical ingredient for various issues. Some claims are legit, whereas others are exaggerated. However, it does help regulate the pH of your skin when used in diluted form. Use it after washing the face.
Take a tablespoon of apple cider vinegar, dilute it with two parts water and get a cotton ball to spread the mixture on your face. Remove it after 20-30 minutes. After a few weeks, you’ll notice a healthy skin with fewer breakouts and diminishing blemishes.
Warning: Never use acidic ingredients without first diluting them with water.
Moisturizer
Most people with oily skin like to wash their face and then rely on their nature-made oil factory (aka excess sebum production) to play the role of a moisturizer. Let’s change that. Carefully chosen moisturizers can prevent the skin from over-drying and restore the pH balance. Opt for a pH-adjusted moisturizer.
Bottom line to skin pH
We hope you finish this article with some great insights into the world of skin pH. To give you a quick recap, here’s a summary. Normal skin pH is anywhere between 4-6, but a value below 5 is better for beautiful skin.
A change in pH, especially to the higher side, results in skin conditions no one wants to have. Keeping your skin pH in the normal range can save you much pain and plenty of dollars.
May your skin be beautiful and radiant.
P.S. Tell your friends to stop using DIY remedies. If they ask, “Why?” then share this goldmine of information!
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605222/
https://pdfs.semanticscholar.org/abc8/34438f45ea67207d524c62ec180413458708.pdf
http://www.scielo.br/scielo.php?pid=S0365 05962002000500006&script=sci_arttext&tlng=en
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1467-2494.2006.00344.xhttps://www.karger.com/Article/FullText/480300







